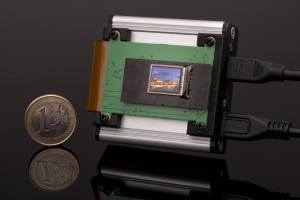
Basler’s camera series designed specifically for the Medical & Life Sciences market is being expanded with additional models: Now eight more color and monochrome models of the Basler MED ace are in series production. The USB 3.0 cameras with 9 MP and 12 MP resolution are equipped with the best CMOS sensor technology and offer frame rates of up to 42 images per second. This means that the number of Basler MED ace cameras has grown to a total of 18 models.
With the certification according to ISO 13485:2016, Basler offers additional and higher quality standards for the production, distribution and service of digital cameras. Manufacturers of medical or in-vitro diagnostic products (IVD) can thus benefit from an effective quality management system with clearly defined standards. Extensive documentation, reliable product quality thanks to a validated and monitored production, traceability and a comprehensive change management support the conformity with international standards and reduce the customer effort required for quality management audits and product documentation. The clearly regulated change management plays a particularly important role for customers. This includes the so-called design freeze, which refers to the fixation of hardware and firmware. This enables Basler to guarantee the product continuity of the Basler MED ace over a long period of time.
The Basler MED ace color and monochrome cameras also impress with their unique Basler MED Feature Sets: Easy Compliance, Brilliant Image, Perfect Color, Low Light Imaging, High Speed and Industrial Excellence. The sophisticated requirements which Basler configured specifically for the Medical & Life Sciences areas combine hardware, firmware and software functions. Their leading benefit is that they help customers to reduce their development effort. They enable images of the highest quality in the shortest possible time, but at the same time offer full flexibility for individual requirements.























 Back to Products
Back to Products