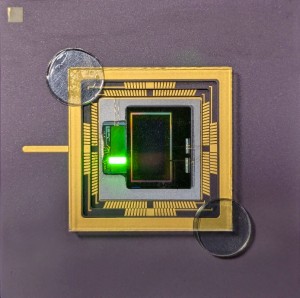
A spectroscopy probe developed at imec enabled surgeon Rutger Schols to compose the first live human surgery spectroscopy atlas. Jose Pozo, EPIC’s CTO, talked to Fokko Pieter Wieringa, Principal Scientist at imec, Netherlands, and learned about the path to bringing medical photonic devices to market.
In 1983, convinced of the potential of medical technology to radically improve patient care, Fokko began a four-year study in Electronics and Medical Technology at the “Noordelijke Hogeschool Leeuwarden” Polytechnics in the Netherlands. After graduating, he worked for 10 years as a Biomedical Engineer at Rijnstate hospital in Arnhem, the Netherlands, which, as he recalls, was great fun as he spent most of his time repairing equipment, improving medical devices, and inventing his first two patents (both using medical photonics).
In 1997, he moved to the Netherlands Organization for applied scientific research (TNO), where he worked as Applied Physics Advisor, for TNO Quality of Life, providing general advice on medical technology, investigating incidents with medical technology and writing international safety and performance standards for medical devices and dosimetry of optical exposure. He also co-authored the EU-Directive on optical radiation safety. Working at TNO was an excellent learning experience because instead of solving problems for one hospital he now had to do this for the whole country. On the technical side, he learned a lot about electromagnetic compatibility, advising on the design of medical devices and installations, commissioning entire hospitals, and CE-marking of medical devices. But most motivating of all was the opportunity to collaborate with driven doctors and patients, i.e., those who were convinced of the benefits of new technologies for improving patient care and treatment.
Frustrated by the need of having to be accompanied by someone with a PhD when he pitched ideas for devices to investors, in 2002, Fokko started a PhD in Biomedical Engineering at Erasmus University in Rotterdam (1 day/week, alongside his job at TNO). A PhD would enable him to talk directly with investors and expand both his technical knowledge and career opportunities.
Upon completion of his PhD in 2007, he was promoted to Senior Scientist Medical Equipment, at TNO Industry & Technology, with responsibility for developing new principles and technologies and improving existing medical devices, while he kept participating in international standardization processes within IEC, ISO and AAMI.
During this period, he also started part-time teaching Biomedical Technology at the Eindhoven University of Technology.
IMEC
In 2017, he became Principal Scientist, at the Interuniversity Microelectronics Centre (imec) in Eindhoven, an international research & development organization, active in the fields of nanoelectronics and digital technologies with its headquarter in Leuven, Belgium. Fokko recalls: “at TNO I helped ASML to solve several technological bottlenecks in the development of Extreme UV (EUV) Lithography, and it is fascinating that this groundbreaking wafer stepper now is standing in the imec cleanroom in Leuven, enabling us to create unprecedented chips that will have a world-scale impact on the future of medical devices. Probably my EUV-related patents will have more overall impact than my directly medical-related patents”. In the photo, Fokko Wieringa is shown in the OR of UMC Maastricht, holding his spectroscopy probe that enabled surgeon Rutger Schols to compose the first live human surgery spectroscopy atlas.
Over the last seven years, Fokko played a key role in the Dutch Kidney Foundations’ NeoKidney initiative to develop a portable artificial kidney. As he explains, although hemodialysis (the current mainstay treatment for kidney failure) keeps people alive, it doesn’t offer a high quality of life as patients are required to visit a dialysis centre 3 times a week for 4 hour treatment sessions. Moreover, the treatment is limited as it only removes 10-15% of the toxins compared with a healthy kidney.
As an alternative, the Dutch Kidney Foundations have partnered with high-tech companies and research institutions to develop a compact, easy-to-use and cost effective dialysis machine that patients can use themselves, directly at home. The advantages over traditional dialysis are enormous: it enables frequent and longer dialysis at home on a time that fits your own schedule (e.g. at night), which limits the treatment’s side effects, such as dialysis hangover and cardiovascular stress, and increases autonomy, quality of life and life expectancy. Moreover, home haemodialysis is considerably cheaper than in-centre treatment.
Reflections on how the barriers against new medical devises can be lowered.
Unfortunately, the success of the portable artificial kidney is an exception rather than the rule, as it is a sad fact that many innovative research projects in medical technologies never reach the clinics. And for those that do, it can take anything up to 20 years before a useable product is in the hands of doctors and patients. Cases in point is Raman spectroscopy and hyperspectral imaging, which although first medically demonstrated in the 1990s, took more than fifteen years before they began to be routinely used for tumor detection.
With more than 33 years MedTech experience and a (co)inventor of 21 patents, and from his experience working with the Dutch Kidney Foundation, Fokko is well placed to offer some recommendations on how the hurdles to market for new medical devices can be lowered:
Consult the end-user to identify the right problem: Asking the right research question means first identifying real needs by listening to doctors, nurses, charity health foundations and patient associations, who have been looking into specific health issues for decades and already know what the real problems are. Once these problems are known, the search can begin for possible technical solutions. Furthermore, if the device is to be used directly by patients themselves, particularly in their own homes, it has to be extremely safe, robust, reliable, and intuitively operational. As a result, human factor engineering is crucially important.
Involve regulatory bodies at the start of the project: By definition, disruptive innovations are not included within existing standards, and to avoid unnecessary delays, government and industry regulatory bodies need to be involved at an early stage of the innovation.
Bring the insurance companies on board at an early stage: No matter how useful a device may be in prolonging life, improving treatment or patient quality of life, it will not commercially succeed without agreement from the state and private health insurance companies to reimburse the costs of using the device. At the end of the day, getting a reimbursement code requires being able to demonstrate not just health benefits but also how the device can reduce the costs of treatment, particularly by reducing time spent in hospital.
Lower the risk of investment: Getting ‘Buy-in’ of patient associations, clinicians, regulatory bodies and insurers significantly reduces the risks for investors and manufacturers, which opens the door for better-termed funding from a much wider variety of sources.
Be careful with technical appropriation by big companies. Most of the truly disruptive innovations come from smaller firms with limited capital. In the medical field, it’s not uncommon for a small company to develop a device and go through the first-in-human trials only to sell out to a much larger company. Although the owners may benefit by being able to pay off their investors and receiving a slice of the cash, in many cases, the new technology is put into cold storage (sometimes never seeing the light of day again) because the larger company doesn’t yet want to disrupt its existing business model while that is still profitable.
More support for small companies: Accordingly, instead of just focusing on investing in research, government funding bodies would get a greater yield if they would also invest in protecting smaller companies from the sharks, for example, by giving start-ups solid advice on making contracts, investment strategies and how to work with regulatory and standards organisations. This increases Societal Return on Investment: In this way public money to fund research & development simply produces a better yield.
Less hype and more roadmaps: Since the dot.com bubble of the 1990s, many entrepreneurs, venture capitalists, consultants, scientists, engineers, and universities have continued to hype-up high-tech. Examples in medical technology abound - from 3D printed drugs and organs to intelligent scalpels. Most recently have been the claims about non-invasive devices that can measure blood glucose for diabetes with no need to prick the skin and draw blood, e.g. skin patches, fingerprint sensors on a mobile phone and contact lens that use fluorescent light to monitor sugars through the eye. Eventually, many of these new horizons indeed are reached, but often later then thought on first sight. As Fokko says: “Hype is like a roller coaster; it goes up and shows exciting new horizons and then we all start screaming when it dives down.”
What’s needed in photonics is a more thoughtful approach and general agreement on a roadmap like the one developed by the semiconductor industry, which competing industries are still sharing because they know it’s to their mutual advantage. Furthermore, this type of coopetition – meaning: collaboration between business competitors in the pursuit of mutually beneficial results – is required across various geopolitical regions, not just at a national level. Afterall, as Fokko points out, if NATO countries can cooperate on the development of a joint strike fighter that’s designed to kill, it’s even more possible to work together internationally to develop medical technology that can save lives.
The future
Implantable or extended artificial organs: Imec has a specific Autonomous Therapeutics program with special interest in chronic diseases particularly kidney disease and inflammatory diseases like rheumatic arthritis. The aim is to use integrated photonics, electronics, fluidics and MEMs to miniaturize complex systems that enable a new generation of implantable or extended artificial organs.
Wearable or contactless medical monitoring technologies: Photonics-based wearable or remote medical monitoring devices can help overcome the problems of discontinuous measurements in hospitals and associated conclusions from doctors based on a very limited amount of data. The idea is to provide a continuous or semi continuous longitudinal data stream to enable clinicians to make better decisions and fine tune treatments while the patients live their daily life. But just as gold diggers are only interested in the gold and not bags of mud, doctors need ways to extract meaningful nuggets of information from truckloads of raw data. This will greatly be enabled by local, on-chip artificial intelligence (so called “tiny AI”) in the same way as our spine reflexes quickly make a lot of routine decisions, and only bother our brains with meaningful events.
Hyperspectral surgical imaging: As surgeon Rutger Schols, one of Fokko’s former PhD students once put it: “Close collaboration with technicians can turn physicians into magicians”. This is nowhere more the case than with camera-based remote optical monitoring of respiration, heart rate and arterial oxygenation together with hyperspectral surgical imaging, which are now entering clinical routine (even in 3D).
If you started again, what would you do differently?
“I’d first study medicine and then engineering. But when I started out, a course like Technical Medicine, which is now offered in the Netherlands by TU-Twente, wasn’t available yet. Nevertheless, I'm very glad having been able to contribute a little bit to help improve the educational possibilities for medical technologists and for technically interested medical doctors.”
What kind of advice would you give the next generation of entrepreneurs?
“First, read articles and watch videos from Milton Chang, who provides very valuable insights into how he learned to become a photonics entrepreneur, including the setbacks and pitfalls”.
“Second, in medical technology, always keep in close contact with patient organizations, driven clinicians, experienced nurses and learn how insurance reimbursement systems work”.
“Third, stay well connected to your life partner, family and friends; they really care for you and give meaning to everything”.
“Fourth, building the team is the most difficult, but most rewarding job. Finding the right team-mates when you begin your entrepreneurial journey, is difficult, but it’s also the key to a life-long friendship”.
“Finally, join an industry association like EPIC so you can take advantage of access to business reports, mentorships and excellent conferences attended by real experts on a variety of topics.”
Written by Jose Pozo, Chief Technology Officer at EPIC (European Photonics Industry Consortium).

































 Back to Features
Back to Features