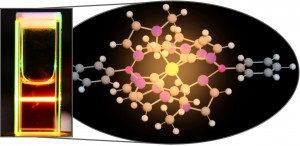
For the first time, researchers at Lund University in Sweden have used advanced molecule design to create an iron molecule that can function as a photocatalyst to produce fuels as well as in solar cells to produce electricity.
The Earth-abundant — and therefore much cheaper — iron could replace the rare and very expensive metal complexes used thus far, such as the noble metals ruthenium, osmium and iridium.
Long live the excited iron complex
We are talking nanoseconds: “A strong ligand field has been shown by us to give long exited-state lifetime of iron complexes — a key factor to obtain photofunctional materials based on iron,” says Chemistry Professor Kenneth Wärnmark of Lund University. His team has now optimized the ligand field strength of the ligands attached to the metal, compared with the previous generation of the complexes, where they had saturated the iron ion with the maximum number of six strongly electron-donating so called N-heterocyclic moieties (that work was described in the journal Nature in 2017). He says this further optimization includes attaching two negative charges to the vicinity of the iron ion and provides a more octahedral ligand field.
Achieving photoluminescence for the first time
What is really new about this advanced iron molecule the researchers created? “Photoluminescence,” Wärnmark replies. “For the first time, iron-based color can be observed by the naked eye at room temperature — light that is emitted from iron!” The scientists explains that until now, the only color seen from iron has been the complementary color that had not been absorbed by the iron, such as in rust, iron-containing rocks, hemoglobins, dyes like Berlin blue or as seen when heating iron until it glows.
“For the first time, the excited state is long-lived enough of an iron complex, so when it encounters another molecule in solution, it has enough time to transfer an electron to that molecule or absorb an electron,” Wärnmark continues, referring to oxidative quenching and reductive quenching, respectively. In other words, for the first time, a bimolecular reaction involving the excited state of iron can take place, which could pave the way to applications of iron complexes in photocatalysis. The expert reckons this breakthrough could “maybe lead to CO2 reductions to the fuel methanol on a large scale, thanks to Earth-abundant iron.”
The novel metal complex created at Lund is quite unique for a metal complex in that light excites an electron from one energy level to a higher energy level, and when the electron returns, it returns directly to the same energy level while simultaneously emitting light — without entering intermediate energy levels like in metal complexes. “In this way, it resembles an organic dye!” the professor concludes.
The new molecule can function as a photocatalyst to produce fuel, and it can one day be utilized in solar cells to produce electricity: “We hope to use the iron complex to produce fuels, such as H2 by water reduction and to methanol by CO2 reduction,” Wärnmark reveals, adding that the advanced design of the iron molecules could also allow its use as dye in dye-sensitized solar cells (DSSCs).
Designing future solar cells with the new iron molecule
This research advance may have formative impact on the design of next-generation solar cells: “The unusual electron configuration of the excited state of iron can be used in p-type DSSCs,” confirms the chemistry professor. “In general, p-type DSSCs are in their infancy. Again, using Earth-abundant metals instead of expensive and rare metals such as ruthenium and iridium.”
Furthermore, in resembling an organic dye, the new iron molecule could help scientists to develop new light-based technologies. Wärnmark mentions the organic dye, for instance, “that is used in polymeric LED, giving a light of a very defined wavelength. This wave length is also of a lower frequency compared to the polymeric LEDs giving new opportunities in color blending.”
How an idea from the field of homogeneous catalysis saved the day
When asked what the biggest challenge was the team faced during the development of this new iron molecule, Wärnmark answers: “To find the right ligand type to increase the ligand field strength in iron complexes to be able to increase the key parameter — the excited state lifetime of the so-called charged transfer state of iron.” How did he overcome this obstacle to eventually succeed? “The idea to use the ligand type N-heterocyclic carbenes I got from the field of homogeneous catalysis, a field in which I have also worked.”
The researcher himself is surprised at the remarkable outcome of the study; at the first-time achievement his team was able to carry through. He was most excited about the fact that “we actually made the iron photoluminescent to the naked eye, something that has been a quest for a long time for many research group — making the second most abundant metal on Earth glowing at room temperature.”
Outlook
Building on their pioneering research success, Wärnmark and his colleagues next plan to make Earth-abundant p-type DSSCs as well as a photocatalyst for the generation of the fuels methanol from CO2 and hydrogen gas from water.
The research work is detailed in the paper "Luminescence and reactivity of a charge-transfer excited iron complex with nanosecond lifetime," published in Science magazine.
Written by Sandra Henderson, Research Editor, Novus Light Technologies Today

























 Back to Features
Back to Features