Researchers, led by Andrew M. Rollins, an associate professor of biomedical engineering at Case Western Reserve University in Cleveland, Ohio (US), looked at a particular type of stress in the heart, known as “shear stress,” which is simply the parallel force of one material sliding along another. In the developing heart, shear stress is induced in the heart’s own endocardial cells as blood cells rush past them. For the first time, the researchers have been able to visualise in three dimensions (3D) the stresses induced by flowing blood in an embryonic heart. Normally, such shear stress helps to control and regulate cellular processes involved in heart development. Even tiny aberrations in the heart beat, however, can alter blood-flow patterns and change these developmental forces, leading to congenital heart defects such as abnormal valve formation.
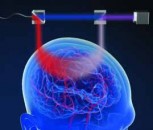
Rollins and his colleagues at Case Western Reserve developed their new imaging method by modifying a technique called Doppler optical coherence tomography (OCT). In OCT, a beam of infrared (IR) light is shown on a tissue and the “echoes” (or reflections) of that light produced at varying depths are used to make an image. The technique, which promises to provide new insight into how and why heart defects develop, is described in a paper titled, “4D shear stress maps of the developing heart using Doppler optical coherence tomography,” and published today in the Optical Society’s (OSA) open-access journal Biomedical Optics Express. The technique has been used to image the interior of blood vessels and is routinely used by ophthalmologists to examine the retina.
In laboratory experiments, Rollins and colleagues directly measured the heart structure and blood flow within the developing hearts of quail embryos. The data was then used to create four-dimensional (4D) images (basically three-dimensional, or 3D, movies), which showed that “locations of high shear correspond with locations of future valve formation,” he says. “Now we are investigating the effects of abnormal shear caused by alcohol exposure on valve development.”
The researchers hope to take what they have learned from these preliminary animal tests and develop a way to apply this technique to humans. The ultimate goal is to develop a tool that doctors can use to decide if early intervention could put a developing heart back on the right track, preventing a defect.
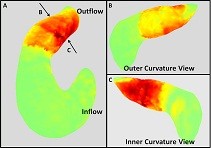
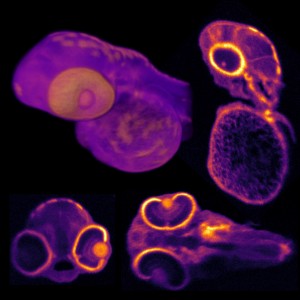
Photo: Shear stress on the inner and outer curvature of the outflow tract. Panel A shows the 3D shear stress map at the time of maximum shear stress in the outflow tract. Panel B and C show the shear stress map of the same heart cropped to show only the outflow tract. Panel B shows the shear stress map oriented to view the outer curvature of the heart tube and Panel C shows the shear stress map oriented to view the inner curvature of the heart. The viewing direction is represented in panel A by the two arrows.

























 Back to News
Back to News