Titanium (Ti) and its alloys have been extensively used as implant materials in orthopedic applications. However, implants may fail due to a lack of osseointegration and/or infection. Researchers endowed an implant surface with favorable biological properties by the dual modification of surface chemistry and nanostructured topography. The application of a nanostructured titanium dioxide (TiO2) coating on Ti-based implants is proposed to enhance tissue-implant interactions while inhibiting bacterial colonization simultaneously due to its chemical stability, biocompatibility, and antimicrobial properties.
Temperature-controlled atomic layer deposition (ALD) was found to provide an effective strategy to produce TiO2 coatings with delicate control of surface nano-topography and surface energy to enhance the interfacial biocompatibility and mitigate bacterial infection.
Design of implant surfaces
In recent years, most research has focused on the design of implant surfaces either to prevent infection while ignoring osseointegration or vice versa. However, for a successful implant, strong osseointegration and anti-infection properties are indispensable, necessitating implant designs to satisfy both simultaneously.
It is clear that both chemical and physical properties of the surface play a major role in modulating the biological events directly at the tissue-implant interface. For Ti-based implants, the naturally formed TiO2 thin layers are thought to act as a barrier between the implant and tissue to prevent metallic ion release into the body, consequently minimizing the chances of an immune response. However, studies indicate that this few-nanometer-thin layer does not provide sufficient long-term corrosion protection. Understanding the role of this native TiO2 film on biomedical Ti implants paves the way for in-depth studies focused on developing a multi-functional Ti implant surface by modifying and structuring the natural oxide layer to further improve biocompatibility.
TiO2 in the form of coatings has also been extensively exploited for antibacterial activities due to photocatalysis, demonstrating promising potential in fighting infections without sacrificing osseointegration. Additionally, it has been reported that the physical topography and structure of the implant surfaces can be optimized down to the nanoscale to produce favorable surface properties (e.g., surface roughness, surface wettability, surface energy, surface phase, etc.) to promote desirable cell functions while minimizing bacteria colonization. Taking all of these into consideration, functionalizing Ti implant surfaces via deposition of a thin, nano-TiO2 coating presents an attractive way to tailor the bioactivity of implant material and hence the osseointegration and disinfection of the implant.
Selecting an appropriate deposition technique
Various techniques have been employed to prepare TiO2 coatings, including e-beam evaporation, sputtering, sol-gel spinning, pulsed laser deposition, chemical vapor deposition and ALD. In selecting an appropriate deposition technology for this application, we considered several criteria such as coating morphology, deposition rate and coverage, interfacial quality and industrial applicability.
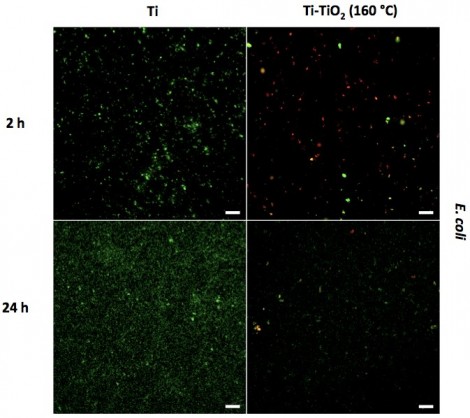
Fluorescent micrographs of E. coli cultured for 2 hours and 24 hours on Ti and Ti-TiO2 (160 °C) samples. Live bacteria are stained green and dead bacteria are stained red. Micrographs show that ALD TiO2 coatings reduce the number of live bacteria
The ALD process is based on the sequential use of self-terminating surface reactions, which is perfect for the deposition of metal oxide layers with atomic layer control on geometrical nanostructures with high aspect ratios. Compared with other more common techniques, ALD possesses several advantages in the deposition of TiO2, such as precise thickness control, extremely conformal coating for nanostructures, excellent large-area uniformity, strong bonding strength, low growth temperature, good reproducibility and straightforward scale-up, applicable to sensitive substrates (i.e., biomaterials).
Numerous studies have reported the formation of homogeneous amorphous films at relative low temperatures and rough surfaces at high temperatures. Therefore, in this study, nano-TiO2 thin films were deposited on commercial Ti substrates by ALD. The effect of surface morphology and structure of the TiO2 layer at different temperatures were investigated. The antibacterial properties of the as-grown TiO2 thin films against different types of bacteria were determined and the cellular biological effects with osteoblasts and fibroblasts were studied in vitro. Additionally, the role of surface wettability/surface energy to changes in specific protein adsorption and biological activities (e.g., cell adhesion and bacteria growth) were correlated.
Surface wettability and surface energy
Surface chemistry together with surface topography can affect surface wetting properties, surface energy and further affect the absorption of proteins to the implant surface, which act as the key factor in mediating cell and bacteria activities at the tissue-implant interface. Therefore, contact angle measurements were carried out to determine the surface wettability and surface energy of the Ti-TiO2 and Ti control samples. As a whole, the data taken from these experiments indicated that all the ALD-TiO2 coated surfaces were slightly hydrophilic, and the total surface energy was relatively higher compared to the Ti control surface.
Antimicrobial assays
The effect of these coatings on the activities of different types of bacteria (Gram-positive bacteria, Gram-negative bacteria and antibiotic-resistant bacteria) was determined. In vitro bacterial results indicated that the Ti-TiO2 samples, specifically Ti-TiO2 (160 °C), inhibited the adhesion and growth of all three types of bacteria (S. aureus, E. coli, and MRSA) significantly (exceeding 80 percent) compared to the results obtained from the Ti control (shown for E. coli in Figure 1). This was accomplished without using antibiotics. Additional fluorescent microscopy experiments to distinguish between live and dead bacteria were also carried out.
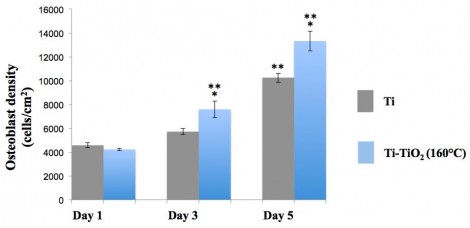
Figure 1: E. coli growth on Ti samples with different TiO2 coatings after 24 hours of culture.
Cell assays
Since osteoblasts are bone-forming cells, a successful orthopedic implant should promote the initial adhesion and proliferation of osteoblasts on the implant surfaces. However, if the body encapsulates the implant by the formation of a soft fibrous tissue and tries to separate it as much as possible, this is the sign of an unsuccessful implantation. Orthopedic implants should be able to minimize fibrous tissue formation to maximize new bone generation. In other words, the modified Ti implant in our study should be able to promote osteoblast functions while suppressing fibroblast functions.
In vitro cells results indicated that initial osteoblast adhesion (after four hours of culture) on Ti-TiO2 samples was doubled relative to that measured from Ti control samples). In our previous antimicrobial study, Ti-TiO2 (160°C) samples showed the best antibacterial efficiency towards all types of bacteria, thus, Ti-TiO2 (160°C) and Ti control substrates were chosen to investigate the effect of the ALD-TiO2 coatings on osteoblast proliferation. As a result, after five days of culture, osteoblast cell numbers on Ti-TiO2 (160°C) samples were 50% higher than those measured on Ti control samples (Figure 2). This enhanced osteoblast cell adhesion, and proliferation can be explained by the increased surface nano roughness and surface energy of the TiO2-coated samples which allows the osteoblasts to adhere in greater numbers due to the higher cell-surface interactions.
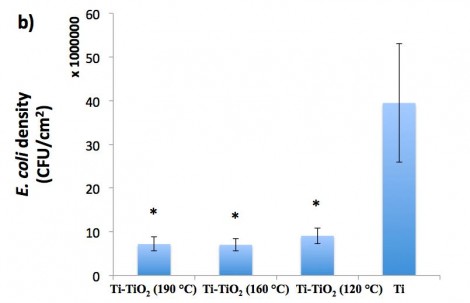
Figure 2: Osteoblast proliferation on Ti and Ti-TiO2 (160 °C) samples.
Promising antimicrobial efficacy and enhanced biocompatibility
"ALD has proven through our experiments to create unique nanoscale surface features that can increase tissue growth (such as bone) and decrease infection - all without the use of drugs. It is a quick and easy way to transform any medical device that we have studied so far to outperform goal standards in the field," said Thomas J. Webster Department of Chemical Engineering, Northeastern University, Boston, Massachusetts (US).
Nanostructured TiO2 coatings on Ti-based implants using ALD showed promising antimicrobial efficacy. In addition, it was found that the TiO2 coating stimulated osteoblast adhesion and proliferation while suppressing fibroblast adhesion and proliferation compared to uncoated materials. Mechanistically, data revealed that the increased protein (casein) adsorption, due to the increased surface nano roughness and surface energy, contributed to these antibacterial properties. Additionally results suggested surface energy as another important factor in determining subsequent cell/bacteria responses could be modulated to certain values (~38.79 mJ/m2) to achieve a variety of desirable biological functions simultaneously. In all, owing to the enhanced biocompatibility and antibacterial activity without using pharmaceutical agents, there is strong potential to apply this ALD-TiO2 coating to the field of orthopedic implants.
Webster concluded that, "By mimicking the natural surface roughness of tissues themselves, ALD provides a quick and easy way to improve tissue growth and decrease infection without resorting to the use of drugs. As our studies expand, we continue to see great promise for the use of ALD in the medical device industry."
This article is based on a technical paper published in the International Journal of Nanomedicine. The complete original paper is available here.
Written by Luting Liu, Wenzhou Medical University; Ritwik Bhatia, Veeco Instruments; and Thomas J. Webster, Northeastern University.





















 Back to Features
Back to Features