The typical approach to generating images of biological samples through a two-photon non-linear process historically means using tunable solid state laser sources (Ti:Sapphire) that are costly and often suffer with reliability issues. In this application note we illustrate the suitability of using a fixed wavelength laser for two-photon microscopy in order to image a wide range of cells with various labels.
Two-photon fluorescence microscopy
Two-photon fluorescence microscopy has become a powerful tool to study biological functions in vivo. In comparison to many standard imaging techniques it has become vital in a range of areas, including research into heart function, liver function, kidney function and especially in optogenetics, where it enables deeper imaging in highly light scattering brain tissue with reduced photobleaching and improved spatial resolution. The main advantages of two-photon fluorescence microscopy is that it enables excitation with a source, which scatters less and only excites samples in the focal plane, thereby providing better spatial resolution.
While this form of microscopy is traditionally enabled using a tunable solid state laser, such sources often fail to generate consistent powers as they are tuned from one wavelength and back to the original wavelength. This can often distort the quality of the data and the image being acquired.
Therefore, it is highly desirable to determine if a fixed wavelength laser source can deliver comparable or better results than a legacy tunable source. This requires a highly stable femtosecond laser, which provides the required combination of pulsewidth, pulse energy and average power at a wavelength that can excite a number of different fluorescent markers,ideally at longer wavelengths, where high-resolution imaging of biological samples can be achieved at depth.
To assess its two-photon fluorescence microscopy capabilities, a range of samples were imaged using an ultrafast fixed 1040 nm wavelength ytterbium fibre-based laser source (the Chromacity 1040).
Imaging of Invitrogen FluoCells, kidney, liver and collagen
The fixed 1040 nm source was used as the excitation laser to selectively highlight several different markers in a range of samples. Unlike tunable solid state lasers, which can produce beams with an elliptical cross-section, the fixed wavelength beam originates from a single-mode fibre, so it is perfectly symmetrical, making it suitable for coupling into commercial laser-scanning microscopes to provide high quality beam shape and high average powers at the sample plane.
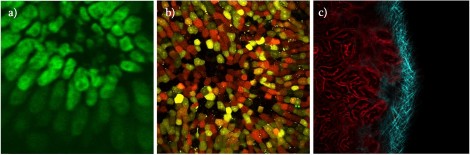
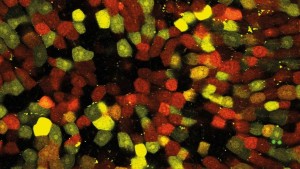
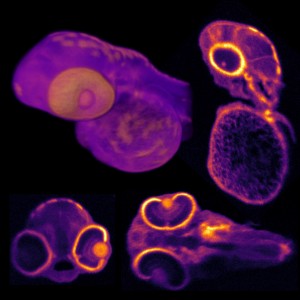
Figure 1: Fluoresced cells
Figure 1 shows typical images acquired from the system when imaging; Fig. 1(a) - Invitrogen Fluocells - section of mouse intestine (cells approximately 5 µm in diameter) via excitation of Sytox Green; Fig. 1(b) demonstrates liver cells imaged using direct excitation of RFP, YFP and GFP using the same excitation laser (images overlaid); and Fig. 1(c) which shows kidney cells overlaid by collagen and demonstrate the ease with which samples can be differentiated using mT:mG as a marker and SHG from the collagen fibres (making it easy to differentiate between cell types).
In the experiments above, the power levels from the 1040 nm source were demonstrated to be more than adequate for generating these fluorescence images, which were recorded with only 150 mW incident on the galvo-scanning mirrors. The fixed wavelength source produced a free-space beam that coupled well with the microscope systems used. Additionally, the source output is horizontally polarized, making it suitable for two-photon polarization microscopy (including second harmonic generation microscopy). It was also insensitive to small amounts of light reflected back from the microscope, so an optical isolator was not required between the laser and the microscope, thus minimizing the losses within the optical system.
Experimental setup
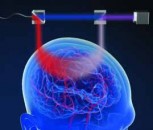
Using the 1040 nm laser source, the images were acquired using a Nikon microscope, into which the beam was introduced via a pair of galvo-scanning mirrors. A Nikon 60x Plan Apochromat oil immersion objective (NA 1.4, working distance 0.21 mm) was used to image the samples. A 520-nm notch filter (Semrock Brightline FF01-520/15-25) filtered out the two-photon fluorescence signal, which was collected using a standard photo-multiplier tube (PMT). Fig. 2. illustrates the setup used.
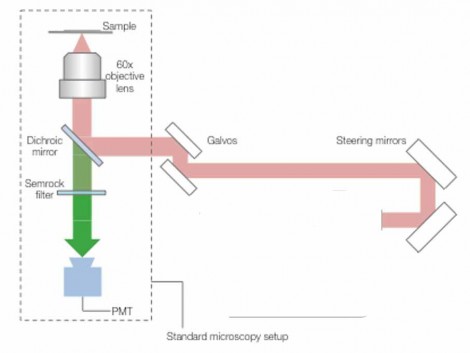
Figure 2 Experimental setup. Source: Chromacity
Generating clear, high-resolution images
Including a fixed wavelength 1040 nm femtosecond laser as part of a multi-photon imaging system allows users to generate clear, high-resolution images that compare favorably with images produced using expensive tunable solid state lasers. This is a result of high beam quality, ultrafast pulses and high average power levels delivered by the 1040 nm source.
This work was carried out in partnership with Chromacity Ltd., ICFO - The Institute of Photonic Sciences, Barcelona, and The Institute of Genetics and Molecular Medicine at the University of Edinburgh.
Written by Christopher Leburn, Co-founder and Commercial Director at Chromacity



























 Back to Features
Back to Features