Researchers have developed a new optical coherence tomography (OCT) approach that can directly image coordination of tiny hair-like structures known as motile cilia in their natural environment. The ability to observe cilia dynamics in living organisms gives researchers a powerful new tool to investigate how these structures move cells and substances through the female reproductive system, as well as other functions of cilia throughout the body.
“Our new method has the potential to answer the longstanding question about cilia's role in the normal physiology of the fallopian tube and the specific functional consequences of ciliopathies — abnormal formation or function of cilia— which have been clearly implicated in ectopic pregnancies, for example,” said research team leader Irina V. Larina from Baylor College of Medicine. “It also has the potential to be further developed and adapted for diagnostic purposes in clinics.”
In Optica, Optica Publishing Group’s journal for high-impact research, researchers from Baylor College of Medicine and Stevens Institute of Technology describe their new imaging approach, which is based on the analysis of pixel intensity fluctuations in OCT images. They used the technique to map and quantify cilia location, beat frequency, and motile coordination through tissue layers in the fallopian tube of a living mouse with micro-scale spatial resolution.
“Although motile cilia play a critical role in many of our body’s systems, their small size makes it very challenging to image their dynamics,” said Larina. “Our new method gives access to this previously inaccessible physiological process. We are excited to see what it will reveal about specific cilia roles in female reproductive physiology and specific links between ciliopathy and infertility.”
In addition to lining the interior surface of the fallopian tube, cilia beat in coordination to propel fluids, mucus and cells along the surfaces of a variety of organs, including the lungs, brain and digestive tract.
Studying cilia in their natural environment
The new research is a part of a larger project investigating female reproductive physiology through advancements in optical imaging. Over the past few years, the research team has developed an intravital OCT imaging approach — meaning it can be used with live mice — as well as various functional OCT methods. These approaches uncovered unexpected behaviors that contradict current views in the reproductive community and suggested new roles for cilia and smooth muscle contractions.
To delve deeper into the specific roles of motile cilia in the fallopian tube, the researchers wanted to investigate cilia coordination and the cilia metachronal wave — a wave-like motion that is produced when each cilium in a group moves in a coordinated pattern — in its physiological environment. However, ciliary dynamics are typically analyzed using high-speed video microscopy of exposed ciliated surfaces, which usually involves dissecting the organ of interest and exposing the ciliated surface for imaging.
To overcome this challenge and allow cilia to be imaged in living organisms, the researchers developed a new OCT method to measure the light intensity fluctuations produced by cilia. OCT is ideal for this because of its microscopic resolution and fast imaging speed along with its ability to acquire volumetric information from up to 2 mm deep in an optically scattering medium such as tissue.
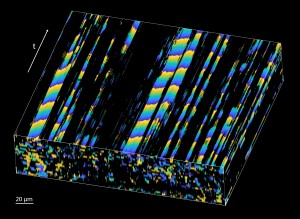
The new method leverages the fact that coherent laser light interacting with tissue will scatter and produce speckles in the OCT images. If these speckles are moving periodically due to moving cilia, it produces a periodic flickering in the corresponding pixels. The researchers developed an image processing method that finds pixels with significant periodic components and then maps the phase of the dominant frequency — which corresponds to the cilia beat frequency — over space and time. This allows visualization and quantification of the metachronal wave created by cilia.
The researchers also built an imaging system that was uniquely tuned to image the fallopian tube of a living mouse and combined it with the intravital mouse OCT imaging approach they previously developed.
Measuring the metachronal wave
After validating the new method, the researchers used it to investigate the effect of temperature on cilia metachronal wave velocity. They found that with increasing temperature not only does the ciliary beat frequency increase — which was known — but the velocity of the metachronal wave also increases. They also performed in vivo imaging of cilia metachronal waves within the mouse fallopian tube using direct volumetric cilia metachronal wave mapping to reveal wave propagations in any direction.
The researchers say that the new method could be modified for use in a variety of studies of cilia dynamics in different organs. With further development, it could also be used with clinical OCT endoscopes to potentially enable OCT imaging of the upper airway for diagnosing problems with coordination of ciliary activity in the respiratory system.
In terms of clinical translation in the field of reproduction, it might be possible to integrate the new approach with fiber-based OCT probes that can be guided through the fallopian tube. These are currently undergoing clinical trials and will likely become standard in clinical practice within a few years.
“The female reproductive system is severely understudied in comparison to other organs,” said Larina. “In addition to the direct scientific impact of the new method, we hope that this study will stimulate other photonics engineers toward the investigation of women’s reproductive physiology and fertility failures.”
Paper: T Xia, K. Umezu, D. M. Scully, S. Wang, I. V. Larina, “In vivo volumetric depth-resolved imaging of cilia metachronal wave with dynamic optical coherence tomography,” 10, 11 (2023).
DOI: 10.1364/OPTICA.499927.






















 Back to News
Back to News