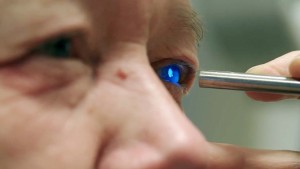
Researchers have developed a simple and fast way to perform optoretinography, an imaging technique that measures light-induced functional activity in the eye’s retina, the network of neurons in the back of our eyes responsible for detecting light and initiating vision. More than 50 percent of people in the U.S. over age 60 are affected by retinal diseases such as macular degeneration and diabetic retinopathy. These diseases affect the retina’s function in ways that reduce eyesight and can progress to blindness if not treated. The new approach could help accelerate the development of new treatments for eye diseases.
“Optoretinography has typically used very costly equipment that required multiple experts to operate while also producing enormous data volumes requiring extensive computational resources,” said research team leader Ravi Jonnal from the University of California, Davis. “We devised a way to do it more cheaper and quicker.”
Jonnal and colleagues report their new approach, which they call velocity-based optoretinography in Optica, Optica Publishing Group’s journal for high-impact research. They also demonstrate the method’s ability to measure retinal response in three healthy subjects.
“Although velocity-based optoretinography could potentially provide clinicians with more accurate and earlier information about functional losses in the retina, its first real impact is more likely to be in expediting clinical trials for new treatments of retinal diseases,” said Jonnal, who performed some of the first optoretinography measurements as a doctoral student in Don Miller’s lab at Indiana University. “If we can detect whether retinal function is getting better or worse faster than with traditional tests such as eye charts, it will greatly accelerate the development of treatments.”
Tracking shape changes
Optoretinography detects slight changes in the shape of neurons that generate or conduct signals in the retina. Until now, Jonnal and other investigators have used adaptive optics and optical coherence tomography (OCT) to visualize and track these neurons in the living, moving eye and then applied motion correction algorithms to stabilize the images and extract the functional response. This costly and time-consuming process requires resolving and tracking the position of individual cellular features and using those positions to determine whether the cell changed shape.
“When we use one of our adaptive optics systems to make optoretinography measurements, the experiment can easily take half a day and result in a terabyte of data that needs to be processed,” said Jonnal. “Processing the data to extract a functional signal takes, minimally, another day or two.”
To avoid the need to resolve and track individual neurons, Jonnal and colleagues wanted to see if they could instead measure the speed, or velocity, at which the retinal neurons move relative to each other. “We believed that even if the positions of the features vary from cell to cell, the speed at which they move relative to one another would be highly correlated among cells,” said Jonnal. “This proved to be correct.”
Measuring moving neurons
To carry out velocity-based optoretinography, the researchers developed a new OCT camera that allows a single operator to gather images from more locations in the retina than is possible with other approaches to optoretinography.
The researchers demonstrated their new technique by using it to collect measurements from three healthy volunteers. They were able to acquire data from each patient in just ten minutes and have that data processed and results available within 2 to 3 minutes. They showed that the functional optoretinographic responses measured with the simple approach scaled with the light stimulus dose used and that the dose-stimulus response was reproducible within and among the volunteers.
They are now planning experiments aimed at demonstrating the technique’s sensitivity to disease-related dysfunction. Jonnal is also working with clinicians at the University of California, Davis to use it for patient imaging and to aid in interpreting results for trials of stem cell therapies and gene therapy treatments for inherited retinal diseases. The researchers would also like to apply the new optoretinography approach to animal models of retinal disease.
Paper: K. V. Vienola, D. Valente, R. J. Zawadzki, R. S. Jonnal, “Velocity-based optoretinography for clinical applications,” Optica, 9, 10 (2022).
DOI: 10.1364/OPTICA.460835





















 Back to News
Back to News