By inserting platinum atoms into an organic semiconductor, University of Utah physicists were able to “tune” the plastic-like polymer to emit light of different colours, a step toward more efficient, less expensive and truly white organic light-emitting diodes (OLEDs) for light bulbs of the future.
Certain existing white light bulbs use LEDs and some phone displays use OLEDs. Neither one is a truly white LED but instead uses LEDs made of different materials that each emit a different colour, then combine or convert those colours to create white light, said University of Utah physicist Z. Valy Vardeny, who led a study of the polymers published online Friday, 13 September in the journal Scientific Reports.
In the new study, Vardeny and colleagues report how they inserted platinum-metal atoms at different intervals along a chain-like organic polymer and thus were able to adjust or tune the colours emitted. That is a step toward a truly white OLED generated by multiple colours from a single polymer.
Existing white OLED displays, like those in recent cell phones, use different organic polymers that emit different colours, which are arranged in pixels of red, green and blue and then combined to make white light, says Vardeny, a distinguished professor of physics.
Vardeny says the new polymer also could be used in a new type of solar power cell in which the platinum would help the polymer convert sunlight to electricity more efficiently. Because the platinum-rich polymer would allow physicists to “read” the information stored in the “spins” of the electrons’ or in their intrinsic angular momentum, the new polymers also have potential uses for computer memory.

Photo 2: University of Utah physicist Z. Valy Vardeny works in a glove box where light-emitting polymers are studied under clean conditions. Vardeny and colleagues have inserted platinum atoms into an organic semiconductor, creating polymers that can be “tuned” to emit light of different colours, a step toward a new kind of organic light-emitting diode, or OLED, that can emit truly white light and be used in future light bulbs. (Photo Credit: Lee J. Siegel, University of Utah)
In the new study, the researchers made the new platinum-rich polymers and then used various optical methods to characterise their properties and show how they light up when stimulated by light.
The polymers in the new study aren’t quite OLEDs because they emit light when stimulated by other light. An OLED emits light when stimulated by electrical current.
Vardeny predicts about one year until design of a “platinum-rich pi-conjugated polymer” that is tuned to emit white light when stimulated by light and about two years until development of truly white OLEDs.
The University of Utah conducted the research with the US Department of Energy’s (DOE) Los Alamos National Laboratory. Additional funding came from the US National Science Foundation’s (NSF) Materials Research Science and Engineering Centre (MRSEC) program at the University of Utah, the National Natural Science Foundation of China and China’s Fundamental Research Funds for the central universities.
Inorganic semiconductors were used to generate colours in the original LEDs, which were introduced in the 1960s. OLEDs generate light with organic polymers that are “plastic” semiconductors and are used in many of the latest cell phones, digital camera displays and big-screen televisions.
Existing white LEDs are not truly white. White results from combining colours of the entire spectrum, but light from blue, green and red LEDs can be combined to create white light, as is the case with many cell phone displays. Other “white” LEDs use blue LEDs, “down-convert” some of the blue to yellow, and then mix the blue and yellow to produce light that appears white.
The new platinum-doped polymers hold promise for making white OLEDs, but can convert more energy to light than other OLEDs now under development, Vardeny says. That is because the addition of platinum to the polymer makes accessible more energy stored within the polymer molecules.
Polymers have two kinds of electronic states:
• A “singlet” state that can be stimulated by light or electricity to emit higher energy, fluorescent blue light. Until now, OLEDs derived their light only from this state, allowing them to convert only 25% of energy into light, which is better than incandescent bulbs but far from perfect.
• A normally inaccessible “triplet” state that theoretically could emit lower energy phosphorescent red light, but normally does not, leaving 75% of electrical energy that goes into the polymer inaccessible for conversion to light.
Vardeny says he and his colleagues decided to add platinum atoms to a polymer because it already was known that “if you put a heavy atom in molecules in general, it can make the triplet state more accessible to being stimulated by light and emitting light.”
Ideally, a new generation of white OLEDs would not only produce truly white light, but also be more energy efficient because they would use both fluorescence and phosphorescence, he adds.
For the study, the researchers used two versions of the same polymer. One version, Pt-1, had a platinum atom in every unit or link in the chain-like semiconducting polymer. Pt-1 emitted violet and yellow light. The other version, Pt-3, had a platinum atom every third unit, and emitted blue and orange light. By varying the amount of platinum in the polymer, the physicists could create and adjust emissions of fluorescent and phosphorescent light and adjust the relative intensity of one colour over another.
Vardeny conducted the new study with former University of Utah postdoctoral researcher Chuanxiang Sheng, now at Nanjing University of Science and Technology in China; Sergei Tretiak of Los Alamos National Laboratory; and University of Utah graduate students Sanjeev Singh, Alessio Gambetta, Tomer Drori and Minghong Tong. The physicists hired chemist Leonard Wojcik to synthesise the platinum-rich polymers.
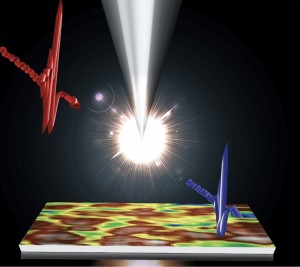
Photo 1 (top of page): A sample of the yellowish-coloured, platinum-rich polymer known as Pt-1 emits light as a laser beam hits it at a University of Utah physics laboratory. The light appears white because the polymer emits a combination of broad-spectrum violet and yellow, which combine to appear white. The polymer and its relatives hold promise for use in a new generation of OLEDs that could produce white light for more efficient LED light bulbs in the future. (Photo Credit: Tek Basel, University of Utah)



























 Back to News
Back to News