“I’m terrified of having my head cut open. While surgery doesn’t frighten me, brain surgery really does. There’s a reason that people say, ‘at least it’s not brain surgery.’ Turns out this saying makes me really nervous. One wrong turn of the scalpel and I could have seizures for the rest of my life, or lose my ability to talk,” said Nicole, a woman who found out a week ago that she has a brain tumor. Neurosurgery is certainly a challenging procedure, for both patients and their physicians. To optimize the chances of success, many tools are employed. These include computed tomography (CT) and magnetic resonance imaging (MRI) machines for pre-operative radiologic scans, and microscopes for live imaging during surgery.
Since the first recorded use of a binocular light microscope during surgery in 1922 [1], microscopes have advanced and have become routinely used in some operations. Beyond improving the view through magnification, resolution and illumination, modern microscopes are also able to integrate digital technologies that differentiate types of tissue. For example, the U.S. regulatory cleared Leica Microsystems FL400 surgical microscope filter that characterizes tumor tissue in neurosurgery. In combination with the active substance 5 aminolevulinic acid (ALA), FL400 fluorescence allows surgeons to visually differentiate malignant glioma tissue from healthy brain tissue in real time. This visualization tool supports decision making in some of the most complex and critical neurosurgical interventions, and aids precise tumor resection, which is vital in order to preserve brain function.
Giving surgeons the ability to operate "heads-up"
The technology of the FL400 fluorescence filter has been designed to provide intense, homogenous excitation light and a well-adjusted observation spectrum. When this is combined with a neurosurgical microscope, such as the Leica ARveo digital augmented reality microscope, surgeons benefit from real-time, bright, and high-contrast delineation of tumor margins. With just a touch of a button, surgeons can view the tissue. This supports the smooth running of an intra-surgical workflow as well as giving surgeons the option to operate "heads-up."
Augmented reality provides real-time images
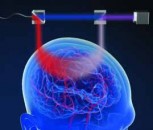
Another challenge for neurosurgeons is the visualization of blood vessels and blood flow. Until relatively recently, blood would need to be visualized using an indocyanine green (ICG) dye that fluoresces under near-infrared (NIR) light. To use this technique, the surgeon would need to pause the operation to watch a separate black and white video, then recall and reconcile this with the anatomic microscope view. However, one of the latest innovations in surgical microscopy is the development of a device that allows the anatomic surgical microscope to produce excitation light and resolve fluorescence emission from the ICG agent. This U.S. regulatory cleared non-contact, non-invasive device, the Leica GLOW800 augmented reality (AR), digitally combines two video streams – one from a camera that captures visible light (VL) images and another from a camera that captures NIR fluorescent angiography information. This produces a high-definition image of cerebral anatomy in natural color, augmented by an overlay of real-time pseudo-colored vascular blood flow, thus providing both angiographic and anatomical information in the same field of view, with full depth perception (see Figure 1).
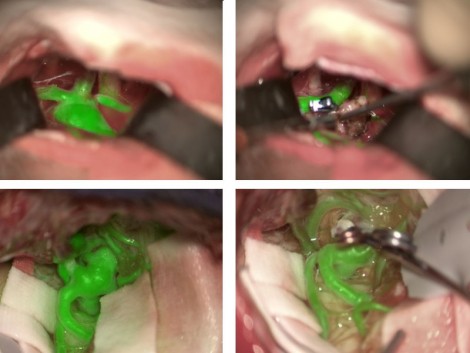
Visualization using Leica GLOW800 AR device to depict blood flow and related tissue perfusion, which can be useful for the placement and checking of an aneurysm clip Source Augmented Reality Fluorescence GLOW800 2
This single view means that surgeons no longer need to pause surgery to watch and recall a black and white NIR fluorescence video, before returning to white light mode to reconcile views and continue operating.
Advances in FLIM hold potential for imaging of cellular metabolism
Fluorescent microscopy, based on the spontaneous emission of light (photons) from excited fluorophores as they decay and drop to their ground (lower energy) state, has come a long way. Its advances have not only improved surgical operations but has also continued to facilitate progress at the frontlines of basic research in the biomedical sciences. One advance in particular continues to evolve and holds great potential for the imaging of cellular metabolism or microenvironmental changes. Fluorescent lifetime imaging (FLIM) is a technique that produces an image based on the time that fluorophores spend in the excited state. It is the lifetime of the fluorophore signal, rather than its intensity, that is used to determine the color of each pixel. It then calculates and represents the image with contrast and definition between materials shown due to different fluorescence decay rates (even if those materials fluoresce at exactly the same wavelength). Fluorescence lifetime has the advantage that it depends on the microenvironment in which the fluorophore is located and more importantly, it is independent of the concentration of the fluorophore. This can be used for making measurements in chemical sensing, such as of the pH of the local molecular environment.
Imaging with fluorescence resonance energy transfer (FRET)
Thus, FLIM is exquisitely suited to report on biomolecular functional states. The duration in which a molecule stays in the excited state is highly dependent on its environment and interactions with other nearby species. As such, FLIM information obtained based on the fluorophore lifetime provides a method of choice for functional imaging. FLIM can also be used to observe and measure Förster or fluorescence resonance energy transfer (FRET) imaging. Imaging with FRET is often used to visualize molecular interactions and in biosensors. FLIM in combination with two-photon excitation can be exploited to study metabolic processes without the need for labelling by analysing the intrinsic fluorescence of nicotinamide adenine dinucleotide (NADH), vitamins and other key biological cofactors. These advantages, such as the independence from fluorophore concentration and the lack of labeling requirement, make FLIM and FLIM-FRET ideal for functional imaging.
Functional imaging goes beyond the traditional recording of the location and concentration of molecular species and enables further investigation of molecular function: their interactions with other biomolecules as well as their activity, conformation, molecular environment and post-translational modifications. Ideally, this must be accomplished at high spatiotemporal resolution, which can be delivered by FLIM. Despite these powerful capabilities, the widespread application of FLIM has been limited. Several factors account for this, such as the intrinsically slow and difficult process required to implement the traditional time-correlated single photon counting (TCSPC) solutions, particularly for complex imaging workflows. As a result, FLIM imaging is currently reserved for use in the specialized laboratory and, even with expert knowledge, traditional TCSPC has been unable to deliver the speed needed to observe biological processes occurring at time scales below tens of seconds [3].
Speed and full integration
To empower scientists with the latest tools and technologies to investigate dynamic cellular physiology in living cells, companies such as Leica Microsystems are continuing to develop microscopy systems and automation, such as the SP8 FALCON (fast lifetime contrast) for FLIM, which is envisioned to be the future of functional imaging. This system is a fully integrated FLIM solution, incorporating the confocal platform. The speed and full integration mean that complex FLIM experiments can now be accessible for life scientists on a daily basis – for many, adding a new dimension of contrast to their imaging and enabling them to perform new kinds of research. Such capabilities include the biosensing of changes in cellular metabolic states and microenvironments, and the tracking of fast molecular interactions between proteins (such as receptor signalling and trafficking).
In the future, FLIM and FLIM-FRET may be more widely applied to better understand disease processes and thus aid in the development and implementation of therapies and other medical interventions. Microscopy solutions are already being applied to detect cancer cells among healthy ones, for example, with multiphoton microscopy in the diagnosis of malignant melanoma [4–6]. Life scientists are also using FLIM-FRET to measure the spatiotemporal dynamics of signaling activity in live neurons [7]. FLIM-FRET is particularly useful for imaging neurons, where light scattering by brain tissue can be problematic for other imaging methods. These are just two examples of specific applications where FLIM and FLIM-FRET offer advantages and possibilities that cannot be realized through other, more conventional confocal microscopy methods. It is anticipated that imaging based on fluorescence lifetimes will continue to garner much novel functional information in the life and biomedical sciences, becoming a standard tool routinely used to investigate biological processes and cellular microenvironments, to provide us with the knowledge and understanding we need to improve human health and create novel treatments and innovative cures for human disease.
Written by Nick Ruszkowski, Global Commercial Strategy Manager, and Giulia Ossato, PhD, Product Manager Confocal, Leica Microsystems
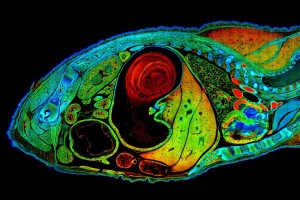
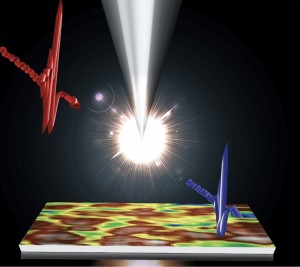
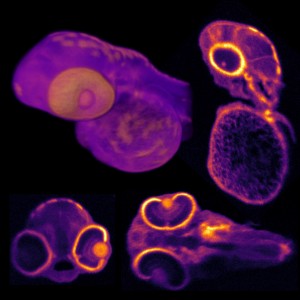
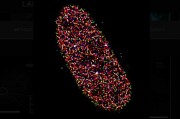
Caption for top image: The picture shows a lifetime image of a mouse embryo recorded with the new SP8 FALCON system. The image was acquired over 722 stitched tiles and fitted to four separate characteristic times. The recording time is about 1 hour – compared to about 1 day with the classical approach. New technologies and new concepts for data evaluation, all implemented in the SP8 FALCON system from Leica Microsystems, render fluorescence lifetime imaging (FLIM) as fuss-free as ordinary confocal imaging.
References:
- Mudry A. The history of the microscope for use in ear surgery. Am J Otol. 2000; 21: 877–86.
- Leica Microsystems. Augmented Reality Fluorescence GLOW800. www.leica-microsystems.com/pro...croscopes/p/glow800/ (accessed November 2019).
- Alvarez LAJ, Widzgowski B, Ossato G, van den Broek G, Jalink K, Kuschel L, Roberti MJ, Hecht F. Application Note: SP8 FALCON: a novel concept in fluorescence lifetime imaging enabling video-rate confocal FLIM. Nature Methods; 2019. www.nature.com/magazine-assets...2473-019-00261-x.pdf (accessed November 2019).
- Yew E, Rowlands C, So PTC. Application of multiphoton microscopy in dermatological studies: a mini-review. J Innov Opt Health Sci. 2014; 7: 1330010.
- Lentsch G, Balu M, Williams J, Lee S, Harris RM, König K, Ganesan A, Tromberg BJ, Nair N, Santhanam U, Misra M. In vivo multiphoton microscopy of melasma. Pigment Cell Melanoma Res. 2019; 32: 403–11.
- König K (Ed.). Multiphoton microscopy and fluorescence lifetime imaging – applications in biology and medicine. De Gruyter. 2018. ISBN: 978-3-11-042998-5.
- Yasuda R. Imaging spatiotemporal dynamics of neuronal signaling using fluorescence resonance energy transfer and fluorescence lifetime imaging microscopy. Curr Opin Neurobiol. 2006;16:551–61.
























 Back to Features
Back to Features