When properly implemented, a piezo-actuated device called a solution switcher allows researchers to investigate fast (millisecond) signaling dynamics involving the binding of agonist molecules like glutamate to cell membrane receptors. In this article, we show why and how it is important to avoid exciting ringing vibrations in the switcher assembly. We then see how Dr. Katie Marwick and colleagues at the University of Edinburgh (Scotland) and University of Montana (US) have used this technology to investigate how a newly identified mutation (GluN2A_N615K) impacts synaptic signaling, confirming its role in a condition called epileptic encephalopathy.
Electrophysiology – directly interrogating membrane activity
Ionotropic pumps and gates such as the N-methyl-D-aspartate (NMDA) receptor are protein assemblies embedded in cell membranes that play a pivotal role in inter-cellular and intra-cellular signaling and in synaptic plasticity – the ability of neurons to vary the strength of networks of connections. There are now multiple techniques for controlling and interrogating this activity, including optogenetics to stimulate activity by opening ion gates using light, calcium dependent fluorophores and other indicators, and traditional techniques such as electrophysiology – all under a microscope often with three-dimensional imaging capabilities, either confocal or multiphoton.
Electrophysiology (patch clamp) remains a frontline tool because of its ability to directly control and/or measure the membrane electrical potential in real time (see for example, Neuroscience Under the Microscope, Novus Light, 21 August, 2017). Here the tip of a micropipette containing an electrode is attached to a cell membrane forming a high impedance seal. There are numerous experimental variations where the sample can range from a whole cell down to small patches containing as little as a single pump or gate protein assembly, as well as “inside-out” configurations with the micropipette attached to the outside of the membrane.
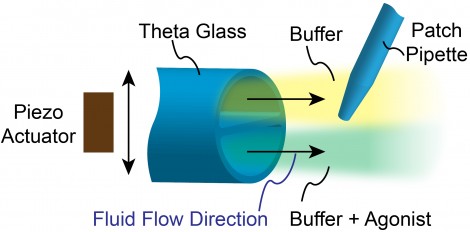
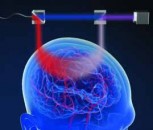
Figure 1: A solution switcher uses a fast PZT actuator to laterally move a micropipette made of theta glass. A patch clamp membrane sample or whole cell is thus rapidly alternated between the two solutions flowing through the theta glass.
Fast solution switching
Electrophysiology provides a unique means to study the response to actual chemical signaling agents (agonists) at neurologically relevant speeds. This is achieved by combining electrophysiology with so-called theta glass and fast solution switching technology. As shown in figure 1, this utilizes a micropipette with a septum where the end of the micropipette has a cross-section like the Greek symbol θ. This has a typical diameter in the 150 – 200 micron range. Two different solutions are then forced to flow out of the end of the theta glass. Typically one solution is a buffer and the other is a buffer plus an agonist like glutamate. The patch clamp tip holding the cell or membrane patch is positioned in these flow streams. Fast lateral motion of the theta glass means the membrane sample is rapidly switched back and forth between the two solutions with the effect on the membrane conductance, recorded by the patch clamp electrode and amplifier.
Because typical cellular responses are in the 200 microsecond range, ideally the speed should be milliseconds or better. The only way to approach this speed is to use a piezo-electric (PZT) flexure guided nanopositioner. An example of this type of high-speed setup is the MXPZT-300 from Siskiyou which provides up to 250 microns of lateral motion with full motion as fast as 6 milliseconds. A unique innovative aspect of this setup merits further examination.
Simplistic actuation of a motion mechanism could present many problematic issues: in particular, the rapid actuation will excite resonances in the pipette, PZT mechanism and the supporting coarse-positioning structure. This can greatly shorten PZT life, and more importantly, the vibrations often manifest themselves as unwanted rapid “switching” as the bores repetitively sweep back and forth due to the vibrations. To eliminate this ringing, the MXPZT-300 is supplied as an integrated assembly with four axes of high precision manual positioning. The use of a standardized assembly means that the system’s resonances have been carefully measured at the factory using techniques such as laser Doppler velocimetry. The PZT driver controller then utilizes a patented Input Shaping® circuit based on a digital signal processor. This coprocessor is factory-configured to nullify the structural vibrations caused by rapid PZT actuation. The nullification targets the specific resonant frequencies characteristic to this standardized setup. The effectiveness of this approach is confirmed in the Doppler velocimetry data shown in figure 2. The result is that any motion profile may be safely commanded by the user. The power supply box is configured to accept inputs from –10V to +10 V. The Input Shaping® coprocessor takes care of vibrations associated with typical square wave inputs, so there’s no need to specify “softer” waveforms.
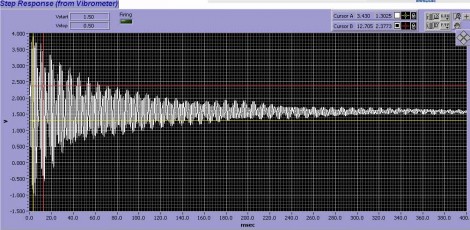
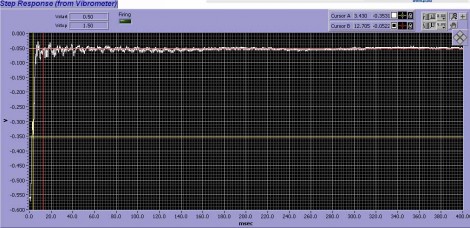
Figure 2. Interferometric position metrology of pipette motion using a laser Doppler vibrometer. The upper trace shows the ringing when the MXPZT-300 actuator is driven by a simple square step. The lower trace shows the effectiveness of using a smart driver programmed to avoid excitation of mechanical resonances in the rig.
Investigating a new NMDA missense variant
In a recently published study [ref], Dr. Katie Marwick and colleagues have used this fast solution switcher to investigate how a recently discovered missense mutation negatively impacts the function of NMDA receptors, a signaling receptor frequently found in neurons. This important protein complex is a heterotetramer; four sub-units overall that comprise two GluN1 and two GluN2 subunits. So far, four different subunits have been cloned (called GluN2A-D). When glutamate binds to NMDA receptors, it allows calcium ions to flow across the membrane. And because this flow can be blocked by magnesium (Mg2+) ions, the “receiving” cell membrane has to be depolarized; thus signaling only happens when the presynaptic cell sends glutamate and the postsynaptic neuron is “listening,” i.e., in a depolarized state.
Marwick, who is also a clinical psychiatrist, explains that, “The large and increasing number of GRIN2A variants (and variants in other genes) found in patients with neurodevelopmental disorders means that functional investigation is clearly required. We have investigated N615K, a heterozygous missense variant found to have arisen de novo in two unrelated people with early onset epileptic encephalopathy. N615K affects a part of the M2 pore helix known to be involved in interacting with Mg2+ ions, and together with other researchers, we hypothesized that this variant underlies the neurodevelopmental disorders in carriers. We set out to explore its functional consequences by electrophysiological analysis in heterologous systems.”
Specifically, the researchers used membrane patches from human (HEK293T) cells to investigate the impact of this variant on NDMA receptors’ conductance when expressed in triheteromers with one wild-type and one mutated GluN2 subunit. (As shown in figure 3, a triheteromer is a NDMA receptor incorporating three different sub-units, whereas a diheteromer is based on just two different sub-unit types.) This combination had not previously been studied, and they reasoned that any insight would be clinically relevant, both because the variant is present heterozygously and because the majority of NMDA receptors in key regions of the adolescent and adult brain are probably GluN1/GluN2A/GluN2B triheteromers. They also investigated whole mammalian neurons using transient transfection to overexpress GluN2AWT and GluN2AN615K subunits in mouse primary cortical neurons.
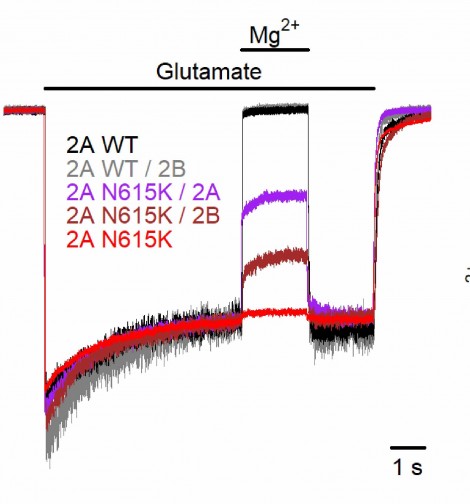
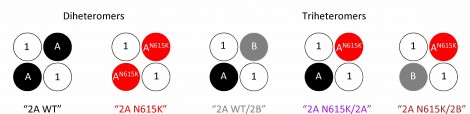
Figure 3. Schematic showing the various NMDA heteromers investigated in this study and the observed differences in magnesium blocking. From Marwick et al, J. Physiol 597.6 (2019) pp 1691-17404
They reasoned that the variant would probably influence both ion permeation rates, i.e., channel conductance, and the Mg2+ blocking function. Their data confirmed that indeed, both of these hypotheses were definitely true – see figure 3. However their data revealed an additional interesting, and as yet unexplained, wrinkle in their understanding of the data. When two copies are present in a receptor, a single low conductance (4X times lower than normal) was seen. But when just one copy is present, surprisingly two conductances are seen: low and intermediate.
Informing genetic counseling
Marwick concludes, “We have used fast solution switching technology to help confirm that the disease-associated variant GluN2AN615K is associated with substantial changes in physiologically crucial properties of NMDA receptors. This information can be used to inform genetic counselling. More generally, these results highlight NMDA receptor-related synaptic transmission as probable candidates for disruption in the pathogenesis of neurodevelopmental disorders.”
Written by John Wingerd, Senior Mechanical Engineer, Siskiyou Corporation
Reference:
Katie F. M. Marwick , Kasper B. Hansen, Paul A. Skehel, Giles E. Hardingham and David J. A.Wyllie, “Functional assessment of triheteromeric NMDA receptors containing a human variant associated with epilepsy,” J. Physiol 597.6 (2019) pp 1691-17404.
























 Back to Features
Back to Features