While optical microscopy methods for neuroscience have expanded significantly in recent times, traditional tools such as patch clamp technology are still widely used because they provide superior data for many types of experiments. Researchers at the AstraZeneca-Tufts Laboratory for Basic and Translational Research are using patch clamp technology to study fundamental aspects of synaptic inhibition that are associated with epileptic-type seizures. This Institute itself is an exciting new business model in the world of neuroscience research, created as a joint collaborative venture between a leading academic institution and a highly respected pharmaceutical giant.
Transmission of signals along neurons involves a spike in the electrical potential across the outer membrane, the so-called action potential. Patch clamp technology is the most direct way to study electrical potential changes across a cell membrane and entails strategically placing electrodes on either side of that membrane.
The oldest and most popular method is to insert a micropipette inside the cell, neuron or other study target; electrical measurements are then made relative to a reference electrode located in the external buffer in which the tissue sample is bathed. There are variants in terms of the geometry of the micropipette positioning relative to the cell membrane.
In simple “cell attached” patch clamp studies, the micropipette is placed over a small area of membrane and suction is applied to make an ion-impervious seal. By using a very small (< 1 micron) micropipette aperture, a patch or membrane can be sampled containing anywhere from just a single ion channel protein up to 100s of ion channels.
Specialized circuitry is then used to maintain a set potential difference between the two electrodes, i.e., to clamp the voltage difference, hence the name “patch clamp.” The setup is often used to clamp to a zero potential value, i.e., to temporarily depolarize the membrane. Depending on the type of cell, the current flow (the signal) required to maintain the clamp during an action potential spike is extremely small – in the picoamp to nanoamp regime.
Treating disorders linked to impaired inhibitory brain transmission
Dr. Tarek Deeb is Program Director in a large research group in the AstraZeneca-Tufts lab headed by Professor Stephen Moss of Tufts Dept of Neuroscience and Dr. Nick Brandon, Chief Scientist, Neuroscience, AstraZeneca. Deeb explains, “It is well-known in neurophysiology that healthy function of the vertebrate central nervous system depends on both synaptic excitation and synaptic inhibition. You can think of these as the “on” and “off” switches that define the signaling between neurons. Impaired neuronal inhibition leads to uncontrolled neuron activity (“firing”), which has long been associated with the increased probability of seizure occurrence and heightened seizure severity. My research goal is to develop novel strategies for the treatment of disorders linked to impaired inhibitory transmission in the brain. Specifically, I’m currently studying the impact of KCC2 inhibitors in this context.”
The principal synaptic transmission inhibitor in the brain is gamma-aminobutyric acid (GABA). Its fundamental role is underlined by the fact that GABA receptors are also critical drug targets for anti-convulsants, sedatives and anesthetics, including such well-known drugs as Valium and Zolpidem. These receptors are ligand-gated ion channels that allow chloride (Cl-) ion influx into neurons causing hyperpolarization of the neuron membrane and thereby preventing neuron firing. Deeb notes that, “This fast inhibitory mechanism critically depends on there being a low Cl- concentration in the neurons. In adult mammalian brains, this low Cl- concentration is maintained by a membrane pump called K+/Cl- co-transporter-2, or KCC2 for short. So, normal synaptic inhibition in turn depends on the correct KCC2 function. Our research is looking at precisely how decreased KCC2 transport function impacts seizure event severity. We are conducting these studies at the cellular level using patch clamp electrodes to monitor the behavior of neurons in slices of murine brain tissue in continuously oxygenated artificial cerebrospinal fluid (ACSF). We use brain samples from normal mice and from a strain of genetically modified mice where a point-mutation is known to impair KCC2 action.”
Inducing seizure-like events
There are a number of ways of inducing seizure-like events (SLEs), including placing the slices in ACSF with a zero magnesium content, or introducing chemical agents such as 4-aminopyridine. Researchers like Deeb can observe the onset, duration and intensity of SLEs using patch clamp signals because SLEs are characterized by a so-called tonic-clonic discharge that is similar to EEG recordings of seizures. These SLEs are repetitive patterns of membrane spikes at the millivolt level with high-power synchronous activity at frequencies (>200 Hz) greater than what is normally observed.
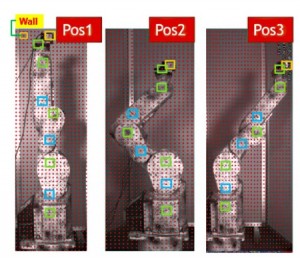
One challenge with patch clamp studies is to accommodate up to four electrodes with 4-axis control in the confined sampling space of an optical microscope. Image courtesy of Matthew Kelley.
One of the practical challenges in conducting this kind of study is the need for stable and repeatable positioning of the micropipette electrodes. In the work discussed here, and in previous studies dating back nearly 15 years, Deeb has used MX7000 micromanipulators from Siskiyou. These use DC servo (MX7000 series) actuators together with crossed-roller bearing stages, since this automated combination delivers high resolution and virtually no drift. DC servos are ideal for low experiments with low signals because they do not draw current when holding a fixed position, unlike steppers and piezo-electrics. Deeb notes that, “Our experiments can span many minutes, so ultra-low drift is very important. Moreover, we often need to change the electrodes, which have a 1 µm tip and are designed for one time use. The ability to press a single button and return to a pre-determined xyz position with micron repeatability is a definite advantage. We also benefit from the 4 axis construction of these micromanipulators, where the electrode axis has its own actuator rather than being ‘synthesized’ from motion in the other three axes.” Some of Deeb’s experiments involve the daunting challenge of placing and adjusting four different electrodes – all within the confines of an upright or inverted microscope. He cites the low profile of these micromanipulators as a critical enabler in this regard.
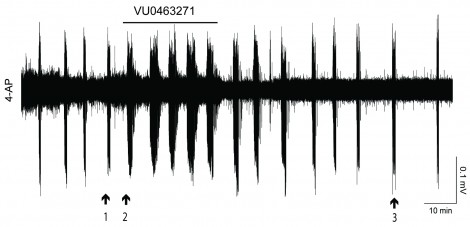
These field potential recordings from the Astrazeneca-Tufts laboratory study showed that when epileptiform events are induced by 4-aminopyridine, the duration of these events is increased by exposure to VU0463271 at 100 nM concentration. Reproduced with permission from Neuropharmacology, 2016 September; 108: 103-110.
It is estimated that up to 1% of the world’s population suffers from temporal lobe epilepsy (TLE). These experiments into fundamental questions into KCC2 inhibition are still ongoing and can hopefully lead to a deeper understanding of epileptic seizures, and possibly thence to improved treatment modalities. In a paper recently published in Neuropharmacology [Kelley et al., 2016], Deeb and co-workers have already shown that impairing KCC2 transport – by either genetic modification or an experimental pharmaceutical agent called VU0463271 – increased the duration of SLEs – see figure above.
Reference: Neuropharmacology, 2016 September; 108: 103–110.
Compromising KCC2 transporter activity enhances the development of continuous seizure activity, M.R. Kelley, T.Z. Deeb, Nicholas J. Brandon, J. Dunlop, P.A. Davies, and S.J. Moss.
By John Wingerd, Siskiyou Corporation























 Back to Features
Back to Features