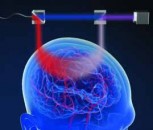
Advancements in imaging and computer science have enabled development of many types of medical image processing systems for the diagnosis of breast cancer. A new type of system from San Antonio, Texas-based Seno Medical Instruments is soon to arrive on the market that -- according to recent clinical trials -- could enable clinicians to diagnose cancer with a greater degree of specificity than ever before. Unlike previous systems used to diagnose breast cancer, the Imagio breast imaging system uses opto-acoustic technology fused with a more conventional ultrasound (OA/US) technique that enables it to generate not only anatomical, but also functional, real-time images of the breast as well.
According to Steve Miller, Seno Medical Instruments’ Senior Vice President of Engineering, earlier diagnostic imaging techniques produced anatomical images that highlighted the purely mechanical differences in the properties of breast issue. But, he says, Seno’s new imagingsystem can also identify two functional hallmarks of cancer. Notably, these are the presence of abnormal blood vessels produced by malignant tumors that help supply nutrients to and remove wastes from a tumor, and the relative reduction in oxygen content of blood that occurs in cancer compared to normal tissue.
“By doing so, the new system provides a more effective tool to help radiologists confirm or rule out malignancies than current diagnostic imaging approaches, all without exposing patients to the potentially harmful ionizing radiation from X-rays used in mammography systems, or the contrast agents used in MRI systems,” said Mr. Miller.
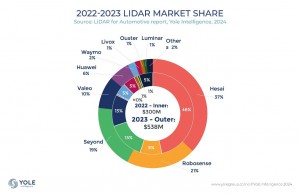
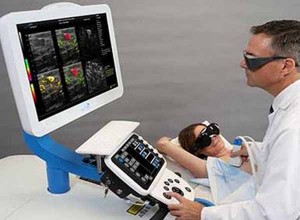
Mobile bedside unit with imaging probe
The system itself comprises two main elements -- a mobile bedside unit that houses a custom processing unit display and keyboard, and a handheld imaging probe positioned over the breast by a clinician. A set of fiber optic cables deliver light at two separate wavelengths from a dual-wavelength short-pulse laser inside the bedside unit to the handheld probe where it is diffused. In addition, pulses of ultrasound are also generated from a transducer housed in the probe, much in the same way that they would be in a more traditional ultrasound machine.
The Imagio system creates a real time six on one image with three independent spatially and temporally co-registered functional images showing distribution of the total hemoglobin concentration and relative distribution of the blood oxygen saturation and one morphological image of tissue structures
According to Bryan Clingman, Vice President of Software at the company, when the two optical pulses of light impinge upon the breast tissue, thermoelastic expansion of the tissue causes ultrasound sound pulses to be emitted from it. The two-wavelength specific backscattered signals are then detected by the transducer in the handheld probe. So too are a third set of ultrasonic signals created by the excitation of the breast tissue in response to the ultrasonic pulses generated by the transducer.
“By combining opto-acoustic imaging and traditional ultrasonic imaging in such a novel manner, the system can employ just one ultrasonic transducer to detect all three ultrasound pulses and use the same set of analog and digital electronics to acquire and process both types of signals,” he said.
Producing functional images
Once the analog signals have been acquired by the transducer they are converted into digital signals and processed by a custom PC-based computer embedded inside the Imagio system to remove noise and restore the temporal shape and ultrasonic spectrum of the original signals. The processed signals generated from, and then acquired by, the transducer are used to create a functional image of the breast similar to those seen on a traditional ultrasound, while the two opto-acoustic signals also captured by the transducer are processed to create functional images of the vessels within the breast.
In addition to emitting pulses of light at two distinct wavelengths, ultrasonic pulses are also emitted from the duplex probe. The transducer detects both the morphologic ultrasonic signals reflected from various tissue structures in and around the lesion and the functional opto-acoustic signals. The signals are then processed and co-registered to produce both functional and anatomical images of the breast.
“The wavelengths of the laser light used were specifically chosen to enable the system to determine the relative amount of oxygenated and deoxygenated hemoglobin within blood vessels in the breast and surrounding tissue. For that reason, the handheld unit emits short pulses of laser light at two wavelengths: 757nm wavelength light that is generated is absorbed primarily by deoxygenated hemoglobin, and 1064 nm wavelength light that is absorbed primarily by oxygenated hemoglobin,” said Mr. Miller.
Hence, the two images created by processing the optoacoustic signals highlight the locations in the breast tissue where the hemoglobin is oxygenated or deoxygenated. And by co-registering those images both spatially and temporally with images created from processing the more traditional ultrasound signal, the system can highlight where the blood vessels lie in an anatomical context.
“However, one of the most important aspects of the system is to highlight relative oxygenation levels -- or those areas where the oxygenation state of the blood changes dramatically,” added Mr. Clingman.
This is important, as cancerous tissues are far more metabolically active than normal tissue. As a cancer grows, the vascular network proliferates to deliver an adequate supply of oxygen and nutrients to the cancer in a process known as angiogenesis. The result is an increased supply of oxygenated blood supplied to the cancerous tissue and a region of intensely deoxygenated hemoglobin at the site of the cancerous tissue itself.
Benign or malignant?
While the presence of a malignant tumor may be highlighted by increased hemoglobin concentration and relatively decreased oxygen content, benign growths can be identified by a variable hemoglobin concentration with relatively more oxygenation. Because the system is able to determine both hemoglobin concentration and its relative oxygenation, clinicians have a better chance of making an accurate diagnosis. Backing up that claim are the results of a recent study that demonstrated that the Imagio OA/US breast imaging system does indeed achieve a better specificity than conventional techniques, and may enable clinicians to more accurately determine whether a lesion is benign or malignant, reducing negative biopsies and obviating the need for further tests.
Breast biopsies are commonly carried out after breast diagnostic imaging procedures, but when tissue from such biopsies are examined under a microscope in the laboratory by pathologists, up to 75% are found to be benign. However, a recent study by Dr. Erin I. Neuschler, Assistant Professor of Radiology at the Feinberg School of Medicine in Chicago, demonstrated that such negative biopsies have the potential to be significantly reduced if opto-acoustic ultrasound technology was used to examine the tumor.
Findings from breast screenings are usually classified into a small number of well-defined categories according to a scheme established by the American College of Radiology known as BI-RADS (Breast Imaging Reporting and Data System). If a lesion is classified as a BI-RAD category of lesion 4a, 4b, 4c or 5 after the result of a screening, then a biopsy is performed. If a lesion is a category 3, it is suspicious but it may be unlikely to be cancer. Even so, individuals classified into such a category will more than likely be called back for further imaging after six months.
In this study, using the Seno OA/US system, readers were able to demonstrate that almost 50% of individuals originally classified with a category 3 lesion could be downgraded to a category 2, potentially obviating the need for further follow-up interval imaging. What is more, use of the system also enabled readers to downgrade over 20% of category 4a’s to either 3 or 2, with the potential to avoid a biopsy in many cases.
The Imagio breast imaging system is currently CE marked in Europe. The system is also currently being processed through the US FDA’s Office of Device Evaluation. Although optical and ultrasound devices are both already used clinically, the combination of technologies present in Seno’s opto-acoustic ultrasound OA/US system requires a regulatory approval process reserved for new technologies, which is known as a Premarket Approval. Approval itself is expected this year with sales in the US immediately thereafter.
Written by Dave Wilson, Senior Editor, Novus Light Technologies Today


































 Back to Features
Back to Features